
Drlogy
Healthcare organization
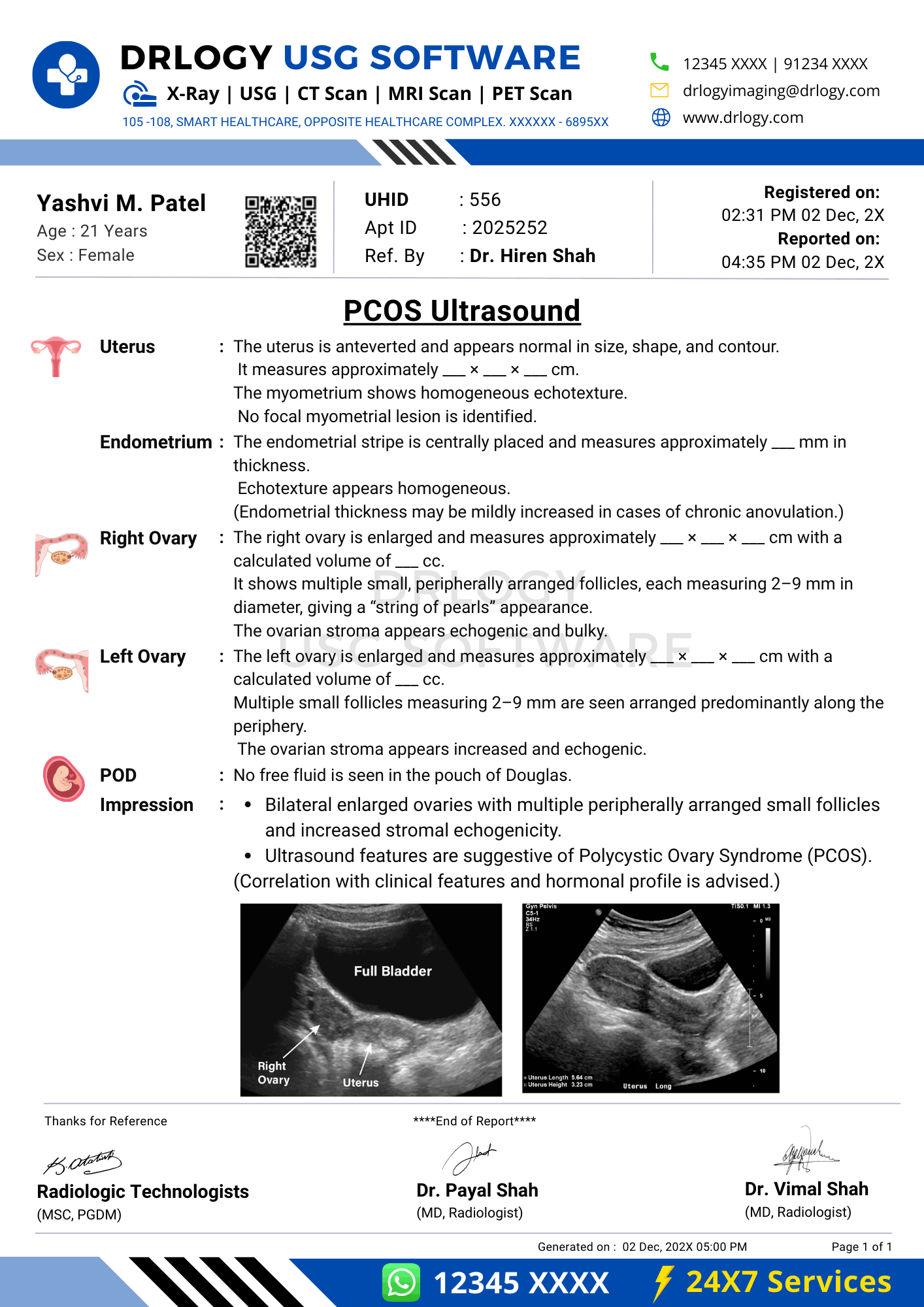
PCOS Ultrasound Report Format for Radiologists
What Is a PCOS Ultrasound Report Format?
A PCOS ultrasound report format is a standardized professional structure for documenting pelvic ultrasound findings relevant to polycystic ovarian morphology using uniform anatomical and measurement terminology.
It supports diagnostic correlation, referrals, longitudinal follow-up, and treatment response assessment when interpreted in conjunction with clinical and biochemical criteria.
It is a medico-legal document defining examination scope, technical adequacy, objective ovarian and pelvic findings, conservative interpretation, and stated limitations.
Check:
Explore the Best AI-Based Ultrasound Reporting Software for Radiologists
Clinical Importance of a Standardized PCOS Ultrasound Report Format
- Diagnostic clarity by systematically documenting ovarian volume, follicle number, distribution, and stromal echogenicity.
- Inter-doctor communication through consistent terminology understood by gynecologists, endocrinologists, and fertility specialists.
- Reporting consistency across radiologists, centers, and follow-up examinations.
- Patient safety by avoiding overdiagnosis and ensuring imaging findings are clearly separated from clinical diagnosis.
- Medico-legal protection by aligning report language with accepted diagnostic frameworks and ultrasound limitations.
A standardized PCOS ultrasound report format improves reliability, comparability, and audit readiness in pelvic imaging practice.
Why Manual Reporting Often Fails to Maintain Standardization at Scale
- Inter-radiologist variability in follicle counting methods, ovarian volume calculation, and descriptive terminology.
- Missed sections in high-volume settings such as incomplete follicle distribution documentation or absent stromal assessment.
- Terminology inconsistency including ambiguous use of terms like polycystic ovaries without measurement support.
- Audit challenges due to narrative reports lacking structured ovarian morphology parameters.
Structured reporting improves consistency and medico-legal robustness while preserving professional judgment.
Indications for PCOS Ultrasound
- Clinical suspicion of polycystic ovarian morphology
- Evaluation of menstrual irregularity
- Assessment in infertility work-up
- Hyperandrogenism evaluation when imaging is requested
- Follow-up of previously documented polycystic ovarian morphology
- Baseline pelvic assessment prior to ovulation induction
Ultrasound findings must always be interpreted in clinical context.
Pre-Examination Details to Be Documented
- Patiententifiers: name, age, sex, unique, accession number, study date and time.
- Referral details: referring clinician, specialty, and specific clinical query.
- Clinical notes: menstrual history, infertility status, prior imaging, hormonal evaluation if provided.
- Preparation status: bladder filling adequacy and imaging approach.
- Safety checks: correct patient verification, pregnancy status confirmation when applicable.
How Reporting Software Ensures Complete Pre-Examination Documentation
- Mandatory field enforcement for indication, menstrual history, and imaging approach.
- Safety checklist compliance for patiententification and pregnancy context confirmation.
- Clinical note traceability linking referral information and prior studies.
- Implementation example: Drlogy Radiology Reporting Software provides structured PCOS-focused pelvic ultrasound templates with compulsory ovarian morphology fields.
Standard Sections of a PCOS Ultrasound Report Format
- Patient & Study Information
- Clinical History / Indication
- Technique / Protocol
- Findings (ovaries, uterus, adnexa)
- Impression / Conclusion
- Limitations of the Study
- Recommendations & Follow-Up (if applicable)
Patient & Study Information Section
This section ensures traceability and accountability:
- Patient demographics andentifiers
- Study date, time, and accession number
- Referring clinician and department
- Examination name and scope (transabdominal and/or transvaginal)
- Comparison with prior studies if available
Clinical History / Indication Section
- Indication for PCOS assessment
- Menstrual pattern and cycle regularity
- Infertility context if applicable
- Prior imaging or treatment history
Documentation must remain concise and clinically relevant.
Technique / Protocol Section
- Positioning: supine with appropriate pelvic exposure.
- Approach: transvaginal ultrasound preferred where appropriate; transabdominal when indicated.
- Views: longitudinal and transverse ovarian and uterine planes.
- Transducer: high-frequency endocavitary probe for optimal follicle visualization.
- Doppler usage: documented if stromal vascularity is assessed.
Technique documentation defines examination scope and limitations.
Findings Section – Organ/System-Wise Reporting
Ovaries
- Laterality and visualization
- Ovarian dimensions and calculated volume
- Follicle number per ovary with size range
- Follicle distribution pattern (peripheral or diffuse)
- Stromal echogenicity assessment
Uterus
- Size and orientation
- Myometrial echotexture
- Endometrial thickness and appearance
Adnexa and Pelvic Cavity
- Presence or absence of adnexal masses
- Pelvic free fluid assessment
Objective description must precede interpretation.
Impression / Conclusion Section
- Summarize ovarian morphology findings
- Use conservative language describing sonographic features
- Avoid definitive diagnostic labeling without clinical correlation
- State that ultrasound findings represent morphology, not standalone diagnosis
Limitations of the Study
- Suboptimal visualization due to bowel gas
- Limited follicle resolution in transabdominal approach
- Patient intolerance of transvaginal examination
- Cycle-related variability affecting follicle count
Documenting limitations ensures medico-legal clarity.
Recommendations & Follow-Up (If Applicable)
- Correlation with clinical and biochemical parameters
- Follow-up imaging only when clinically indicated
- Interval assessment if prior comparison is required
Normal PCOS Ultrasound Report Format (Sample)
Patient & Study Information:
Patient: [Name], [Age]/[Sex]
Study Date: [DD-MM-YYYY]
Examination: Pelvic Ultrasound
Clinical History / Indication:
Evaluation for polycystic ovarian morphology.
Technique / Protocol:
Transvaginal pelvic ultrasound performed.
Findings:
Both ovaries are normal in size and morphology. Follicle count and distribution are within expected limits. Uterus and endometrium appear unremarkable. No adnexal mass or free fluid is seen.
Impression / Conclusion:
No sonographic evidence of polycystic ovarian morphology.
Limitations:
No significant technical limitation noted.
Abnormal PCOS Ultrasound Report Format (Sample)
Patient & Study Information:
Patient: [Name], [Age]/[Sex]
Study Date: [DD-MM-YYYY]
Examination: Pelvic Ultrasound
Clinical History / Indication:
Menstrual irregularity.
Technique / Protocol:
Transvaginal pelvic ultrasound performed.
Findings:
Both ovaries are enlarged with increased follicle number arranged predominantly peripherally. Ovarian stromal echogenicity appears increased. Uterus is normal in size and echotexture.
Impression / Conclusion:
Ovarian morphology as described. Findings should be correlated with clinical and biochemical parameters.
Limitations:
Assessment limited by cycle-related variability.
How Drlogy Radiology Reporting Software Standardizes These Report Formats
- Template-driven reporting for ovarian morphology parameters
- Impression safety controls promoting conservative language
- Uniform formatting across pelvic ultrasound studies
- AI-enabled reporting assistance under radiologist supervision
- Audit-ready documentation for quality assurance
10 Key Clinical Guidelines for an Effective PCOS Ultrasound Report Format
- Document imaging approach clearly.
- Measure and record ovarian volume consistently.
- Count and document follicles systematically.
- Describe follicle distribution objectively.
- Assess stromal echogenicity cautiously.
- Document uterine findings separately.
- Avoid definitive diagnostic labeling.
- Separate findings from impression.
- Document limitations explicitly.
- Correlate imaging with clinical context.
Adherence improves reporting accuracy and medico-legal safety.
Common Reporting Errors to Avoid
- Labeling PCOS without measurement support
- Incomplete follicle documentation
- Omission of ovarian volume calculation
- Overinterpretation of normal variants
- Missing limitation statements
Avoidance of these errors strengthens report credibility.
Medico-Legal Considerations in Radiology Reporting
- Objective documentation of ovarian morphology
- Explicit limitation statements
- Conservative impression wording
- Clear accountability and authorization
- Audit-ready structure
- Appropriate disclaimers
- Accurate comparison with prior studies
Structured Reporting vs Narrative Reporting
| Aspect | Structured | Narrative |
|---|---|---|
| Completeness | High | Variable |
| Consistency | Standardized | Operator dependent |
| Audit readiness | Strong | Limited |
| Efficiency | Optimized | Variable |
| Medico-legal safety | Enhanced | Variable |
Role of Technology in Radiology Reporting
- PACS and RIS integration
- Voice dictation with templates
- AI-assisted formatting
- RIS-based structured templates
- Modality-specific reporting tools
Technology enhances consistency without replacing professional judgment.
Why High-Volume Radiology Centers Prefer Software-Based Reporting Formats
- Faster turnaround time
- Improved quality assurance
- Multi-radiologist consistency
- Enhanced scalability
- Reduced omission errors
- Audit-ready documentation
- Stronger medico-legal protection
Frequently Asked Questions (FAQs)
What defines a standard PCOS ultrasound report format?
A structured format documenting ovarian morphology with conservative interpretation and stated limitations.
Can ultrasound alone diagnose PCOS?
Ultrasound documents ovarian morphology and must be correlated with clinical and biochemical findings.
How should follicle count be reported?
Using standardized counting methods with size range and distribution.
Why are limitations important in PCOS ultrasound reporting?
They acknowledge technical and physiological variability and support medico-legal safety.
Key Takeaways for Radiology Professionals
- Use standardized ovarian morphology documentation.
- Avoid definitive diagnostic labeling.
- Maintain conservative impression language.
- Explicitly document limitations.
Consistent structured reporting improves clinical communication and medico-legal robustness.
Expert Picks
Final Conclusion
A standardized PCOS ultrasound report format is essential for accurate pelvic imaging communication, reliable follow-up, and medico-legal safety when evaluating ovarian morphology.
Structured reporting software supports consistency, completeness, and conservative interpretation while aligning with real-world radiology practice and professional standards.




