
Drlogy
Healthcare organization
USG Abdomen and Pelvis Report Format for Radiologists
What Is a USG Abdomen and Pelvis Report Format?
A USG abdomen and pelvis report format is a standardized professional structure for documenting ultrasonographic evaluation of abdominal and pelvic organs in a consistent and interpretable manner.
Clinically, it supports diagnosis, referrals, follow-up planning, and longitudinal comparison by ensuring systematic assessment of abdominal and pelvic structures.
Medico-legally, it serves as an official medical record defining examination scope, technique, findings, interpretation, and limitations according to accepted reporting standards.
Check:
Explore the Best AI-Based Ultrasound Reporting Software for Radiologists
Clinical Importance of a Standardized USG Abdomen and Pelvis Report Format
Ultrasound of the abdomen and pelvis is one of the most frequently performed imaging examinations in routine clinical practice. It involves evaluation of multiple organ systems, variable patient preparation, and operator-dependent image acquisition. Because of this complexity, standardized reporting plays a critical in maintaining diagnostic quality and professional accountability.
Structured reporting improves diagnostic clarity by ensuring that every abdominal and pelvic organ is assessed and documented in a predefined sequence. This reduces ambiguity and prevents omission of relevant structures such as pelvic organs, urinary bladder, adnexa, prostate, or peritoneal spaces.
Standardization enhances inter-doctor communication by using consistent terminology and report structure that can be easily interpreted by clinicians, surgeons, gynecologists, urologists, and other radiologists. Clear communication is particularly important when imaging findings guide urgent clinical decisions.
Uniform report formats improve reporting consistency across multiple radiologists, shifts, and locations. This is especially relevant in group practices, multispecialty hospitals, and teleradiology environments where multiple reporting doctors contribute to patient care.
From a safety perspective, structured reporting supports patient safety by reducing the likelihood of missed findings, incomplete examinations, or ambiguous impressions that could delay diagnosis or management.
Finally, standardized documentation provides essential medico-legal protection. Clear recording of findings, impressions, and limitations demonstrates adherence to accepted professional standards during audits, peer review, and legal scrutiny.
Why Manual Reporting Often Fails to Maintain Standardization at Scale
Despite professional expertise, manual narrative reporting frequently struggles to maintain uniformity in high-volume diagnostic settings. This is not due to lack of competence, but rather the inherent variability of free-text reporting under time pressure.
Common challenges include inter-radiologist variability, where different reportings lead to inconsistent structure, terminology, and emphasis. Even within the same department, reports for similar findings may vary significantly.
In busy workflows, missed sections are common. Pelvic organs or secondary findings may be inadequately described or omitted entirely, particularly when examination scope is broad.
Terminology inconsistency is another frequent issue. Different descriptors for similar findings can create confusion for referring clinicians and complicate follow-up comparisons.
Manual reporting also poses audit challenges. Free-text reports are difficult to standardize, search, and benchmark during quality assurance and medico-legal review.
Software-assisted structured reporting addresses these issues by enforcing completeness and consistency while preserving radiologist autonomy and interpretive judgment.
Indications for USG Abdomen and Pelvis
Common clinically relevant indications include:
- Acute or chronic abdominal pain
- Pelvic pain or lower abdominal pain
- Evaluation of hepatobiliary disease
- Assessment of urinary tract pathology
- Gynecological complaints and menstrual abnormalities
- Prostate evaluation and lower urinary tract symptoms
- Detection or follow-up of abdominal or pelvic masses
- Assessment of ascites or pelvic free fluid
- Follow-up of known abdominal or pelvic pathology
Pre-Examination Details to Be Documented
Accurate pre-examination documentation forms the foundation of a reliable and defensible report.
Mandatory details include:
- Patient name, age, sex, and uniqueentification number
- Referring clinician, department, and clinical specialty
- Clear clinical indication and relevant medical history
- Preparation status, including fasting and bladder filling
- Safety and procedural verification
Failure to document these elements can compromise interpretation and medico-legal validity.
How Reporting Software Ensures Complete Pre-Examination Documentation
Structured radiology reporting systems enhance documentation quality through:
- Mandatory field enforcement for patiententifiers
- Required entry of clinical indication before reporting
- Integrated preparation and safety checklist compliance
- Traceable linkage between referral notes and imaging findings
Drlogy Radiology Reporting Software may be cited as an implementation example where such documentation safeguards are embedded into daily radiology workflows.
Standard Sections of a USG Abdomen and Pelvis Report Format
A universally accepted report format includes:
- Patient and Study Information
- Clinical History / Indication
- Technique / Protocol
- Findings (organ/system-wise)
- Impression / Conclusion
- Limitations of the Study
- Recommendations and Follow-Up, if applicable
Patient & Study Information Section
This section establishesentity and traceability and must include:
- Patient demographics
- Study date and time
- Accession or study number
- Referring clinician details
Accurateentifiers are essential for follow-up, comparison, and audit readiness.
Clinical History / Indication Section
Best practices include:
- Concise, relevant clinical documentation
- Inclusion of symptoms, laboratory findings, or prior imaging when relevant
- Avoidance of speculative or unrelated clinical information
This section provides essential context for interpreting imaging findings.
Technique / Protocol Section
For USG abdomen and pelvis, documentation should include:
- Patient positioning during examination
- Transducer type and frequency
- Transabdominal scanning approach
- Adequacy of bladder filling
- Use of focused or additional views, if performed
The technique section defines examination scope and limitations.
Findings Section – Organ/System-Wise Reporting
The findings section is the diagnostic core and must be objective, systematic, and comprehensive.
Best practices include:
- Describing observations before interpretation
- Explicitly documenting normal structures
- Reporting abnormalities with size, echotexture, margins, and precise location
- Using consistent, standardized terminology
Commonly evaluated abdominal structures include:
- Liver
- Gallbladder and biliary tree
- Pancreas
- Spleen
- Kidneys
- Major abdominal vessels
- Peritoneal cavity
Commonly evaluated pelvic structures include:
- Urinary bladder
- Uterus and endometrium
- Ovaries and adnexa
- Prostate and seminal vesicles
- Pelvic free fluid
- Adjacent soft tissues
Impression / Conclusion Section
The impression should:
- Summarize key imaging findings succinctly
- Use conservative, non-definitive language
- Include differential considerations when appropriate
- Avoid diagnostic overstatement beyond imaging capability
This section guides clinicians while maintaining medico-legal safety.
Limitations of the Study
Limitations must be documented whenever present.
Common examples include:
- Inadequate patient preparation
- Suboptimal bladder distension
- Bowel gas interference
- Patient body habitus
- Limited visualization of deep pelvic structures
Clear documentation of limitations protects diagnostic integrity.
Recommendations & Follow-Up (If Applicable)
Recommendations should be:
- Clinically appropriate and justified
- Conservative and non-directive
- Clearly separated from diagnostic conclusions
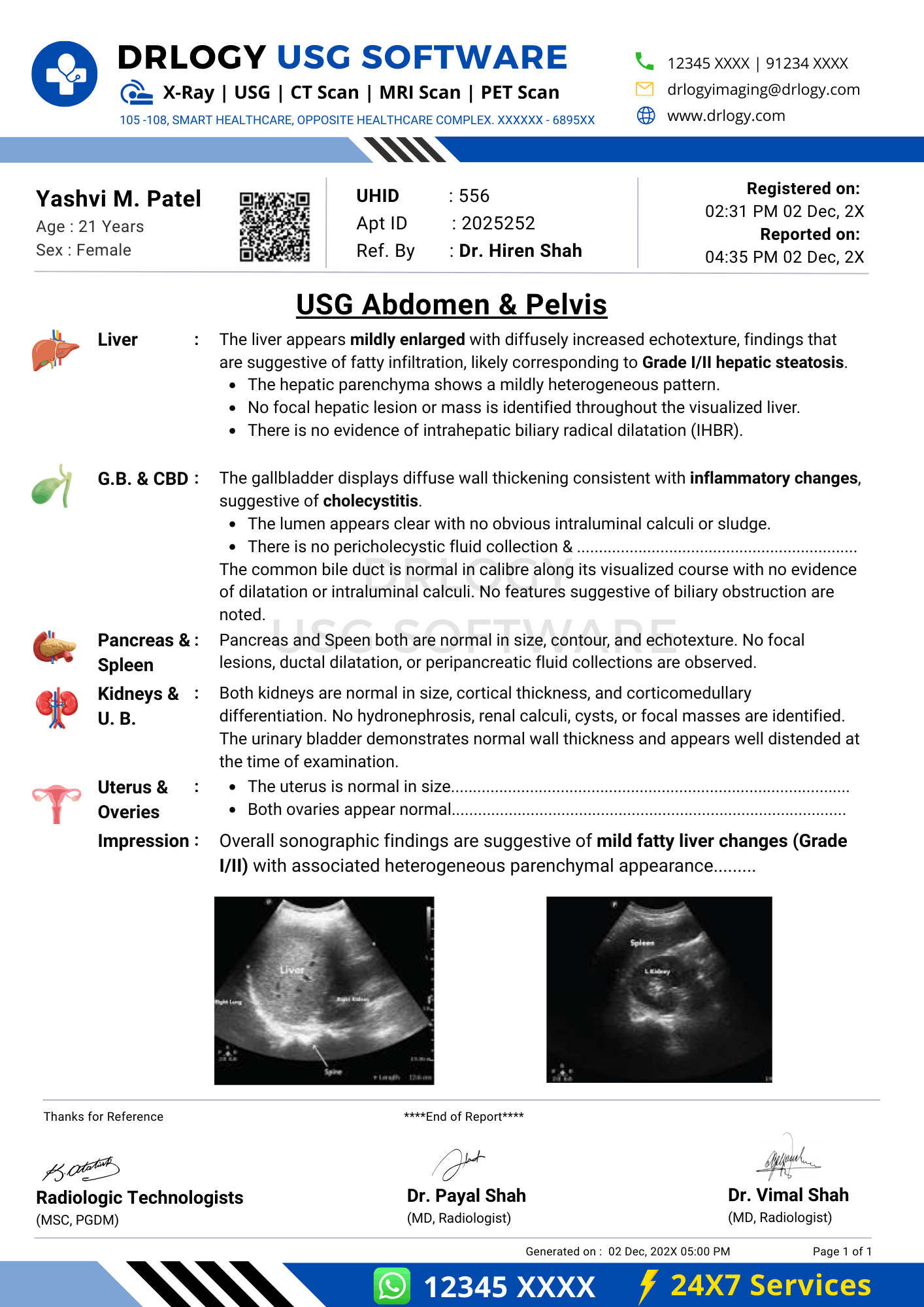
Normal USG Abdomen and Pelvis Report Format (Sample)
Findings:
The liver is normal in size with homogeneous echotexture. No focal lesion is seen. Gallbladder is well distended with normal wall thickness. No calculus or sludge noted. Common bile duct is within normal limits. Pancreas, spleen, and kidneys appear normal. The urinary bladder is adequately distended with normal wall thickness. Uterus is normal in size and echotexture. Endometrial thickness is within normal limits. Both ovaries are normal. No pelvic free fluid is seen.
Impression:
Normal ultrasonography of the abdomen and pelvis.
Abnormal USG Abdomen and Pelvis Report Format (Sample)
Findings:
The liver shows mild enlargement with altered echotexture. Gallbladder demonstrates an echogenic focus with posterior acoustic shadowing. Urinary bladder shows mild wall thickening. A cystic lesion is noted in the left adnexa. Mild pelvic free fluid is present.
Impression:
Ultrasonographic findings as described above. Clinical correlation is advised.
How Drlogy Radiology Reporting Software Standardizes These Report Formats
Clinically relevant standardization mechanisms include:
- Template-driven organ-wise reporting
- Impression safety controls to prevent overstatement
- Uniform formatting across ultrasound and other modalities
- AI-enabled draft generation from imaging workflows
- Audit-ready structured documentation
No pricing, promotional language, or call-to-action elements are included in clinical reporting.
10 Key Clinical Guidelines for an Effective USG Abdomen and Pelvis Report Format
- Maintain consistent section order
- Document all evaluated abdominal and pelvic organs
- Explicitly state normal findings
- Use standardized anatomical terminology
- Avoid speculative diagnostic language
- Separate findings from impressions
- Document limitations clearly
- Verify patiententifiers
- Keep impressions concise
- Support longitudinal comparison
Adherence to these guidelines improves diagnostic quality and medico-legal safety.
Common Reporting Errors to Avoid
- Omission of pelvic or abdominal structures
- Over-interpretation of equivocal findings
- Inconsistent terminology
- Failure to document limitations
- Non-standard impression phrasing
Medico-Legal Considerations in Radiology Reporting
Key medico-legal considerations include:
- Complete and accurate documentation
- Conservative diagnostic language
- Clear accountability of the reporting radiologist
- Explicit documentation of study limitations
- Audit and peer-review readiness
- Secure record retention
- Consistency with accepted reporting standards
Structured Reporting vs Narrative Reporting
| Aspect | Structured Reporting | Narrative Reporting |
|---|---|---|
| Consistency | High | Variable |
| Audit readiness | Strong | Limited |
| Efficiency | Optimized | Operator dependent |
Role of Technology in Radiology Reporting
Technology supports reporting through:
- PACS and RIS integration
- Voice dictation with structured templates
- AI-assisted formatting and consistency checks
- RIS-based structured reporting modules
- Modality-specific reporting software
Why High-Volume Radiology Centers Prefer Software-Based Reporting Formats
Operational benefits include:
- Reduced report turnaround time
- Improved quality assurance
- Consistency across multiple radiologists
- Scalable reporting workflows
- Enhanced audit readiness
- Reduced reporting errors
- Uniform documentation across modalities
Frequently Asked Questions (FAQs)
What is the standard structure of a USG abdomen and pelvis report format?
A structured sequence including patient details, technique, findings, impression, and limitations.
Should normal abdominal and pelvic organs be explicitly documented?
Yes. Explicit documentation improves clarity, follow-up comparison, and medico-legal safety.
How detailed should the impression be?
Concise, summary-focused, and limited strictly to imaging findings.
Is structured reporting mandatory for abdomen and pelvis ultrasound?
Not mandatory, but strongly recommended in professional radiology practice.
Does software-based reporting replace radiologist judgment?
No. Software enhances consistency while preserving clinical expertise.
Key Takeaways for Radiology Professionals
- Standardized formats improve diagnostic reliability
- Structured reporting reduces omissions and variability
- Conservative impressions protect professional integrity
- Technology enhances, not replaces, clinical judgment
Expert Picks
Final Conclusion
A standardized USG abdomen and pelvis report format is essential for accurate clinical communication, patient safety, and medico-legal protection in modern radiology practice.
Structured reporting systems, when aligned with real-world workflows, enable consistency in high-volume environments while fully respecting the radiologist’s professional judgment and responsibility.




